A. Scuiller, A. Dupas, G. Lefebvre, N. Bouriche, H. Chédotal, G. Guillamot, J. Cossy, C. Meyer
Chem. Eur. J. 2025, 31, e202500590
https://doi.org/10.1002/chem.202500590
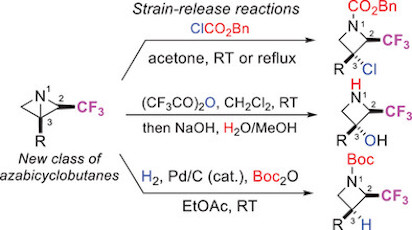
Substituted azetidines are privileged heterocyclic scaffolds in medicinal chemistry and have become synthetic targets of high interest in recent years. With the goal of developing a new access to azetidines incorporating the pharmaceutically relevant trifluoromethyl group, the reactivity of 2-(trifluoromethyl)-1-azabicyclo[1.1.0]butanes was investigated in polar strain-release reactions. By using benzyl chloroformate or trifluoroacetic anhydride as reacting partners, diversely substituted 3-chloroazetidines, 3-substituted azetidines and azetidin-3-ols bearing a trifluoromethyl group at C2 could be readily synthesized. In addition, palladium-catalyzed hydrogenolysis reactions provided an entry to cis-3-aryl-2-trifluoromethyl azetidines.

